The new research, published in the Journal of the American Chemical Society, could help manufacturers produce recyclable, self-healing commercial batteries.
As lithium-ion batteries go through multiple charging and discharging cycles, they form tiny dendrites of solid lithium called dendritic crystals, the researchers said. These structures shorten battery life, create hot spots and short circuits, and sometimes become large enough to puncture the battery's internal components, leading to explosive chemical reactions between electrodes and the electrolyte.
Chemists and engineers have been pushing to replace liquid electrolytes in lithium-ion batteries with solid materials, such as ceramics or polymers. However, most of these materials are rigid and brittle, resulting in poor contact between the electrolyte and the electrode and reduced conductivity.
Brian Jing, co-author of the paper, said: "Solid ionic conductive polymers are an option for the development of non-liquid electrolytes. However, the high temperature conditions inside the cell melt most of the polymer, which again leads to dendrites and failure."
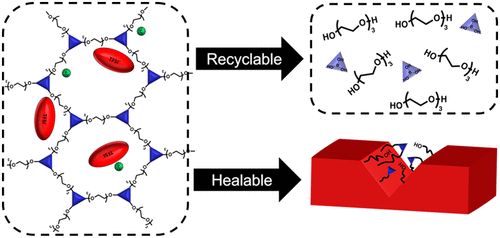
Past research has produced solid electrolytes by using networks of polymer chains that cross-link to form rubber-like lithium conductors, an approach that delays the growth of dendritic crystals, but these materials are too complex to be recycled or repaired once damaged.
To solve this problem, the researchers developed a networked polymer electrolyte in which cross-linking points can undergo exchange reactions and exchange polymer chains. Compared to linear polymers, these networks actually harden when heated, which minimizes dendrite problems, the researchers say. In addition, they readily decompose and re-solidify into a network structure after damage, making them recyclable and, due to their self-healing properties, able to resume conductivity after damage.
"This novel network polymer also shows remarkable properties, namely that both conductivity and stiffness increase with heating, which are not present in conventional polymer electrolytes," says Jing.
"Most polymers require strong acids and high temperatures to break down," said Christopher Evans, professor of materials science and engineering and lead author of the paper. Our material is water soluble at room temperature, which is a very energy efficient and environmentally friendly process."
The team explored the conductivity of the new material and found its potential as an effective battery electrolyte, but they admit there is still some way to go before it can be compared to batteries in use today.
Evans said: "I think this work provides an interesting test platform for others, we used a very special in the polymer chemistry and a very special dynamic key, but we think this platform can be reconfigured, which together with many other chemicals used, to adjust the conductivity and mechanical properties.